Erin Chambers 1 , Mary E. Lame 2 , Diane M. Diehl 2 , Sarah Grimwood 2 and Yanhua Zhang 3
1 Waters Corporation, Milford, MA, 01757; 2 Department of Neuroscience, Pfizer Pharmaceuticals, Groton, Connecticut, 06340; 3 PDM Department, Pfizer Pharmaceuticals, Groton, Connecticut, 06340
introduction
The deposition/formation of insoluble aggregates of amyloid beta peptide (Aβ) in the brain is seen as a key event in Alzheimer's disease (AD). Therapeutic strategies focus on small molecule inhibitors or immunotherapies to reduce or increase the level of amyloidogenic peptide production. Therefore, finding a highly sensitive and reliable quantitative analysis method for amyloid peptides in cerebrospinal fluid to determine its relationship with AD is of utmost importance to many researchers. However, the analysis of these A[beta] peptides is extremely challenging not only because of their relatively low abundance in biological fluids, but also because they may be bound by other proteins and have a tendency to form oligomers.
The determination of these peptides is routinely performed using immunoassays (due to their selectivity and sensitivity) or by lengthy immunoprecipitation followed by SPE. The method development time required for immunoassays is longer than the LC/MS/MS method development time; they require multiple determinations of multiple Aβ peptides and their linear dynamic range is limited compared to LC/MS/MS. Immunoassays have cross-reactive and non-specific binding, requiring the use of expensive antibodies, and the enrichment of samples/species depends on the selectivity of the antibody. The labor intensity of immunoassays is large, and inaccurate determination and matrix interference are also common problems. Therefore, there is a need to develop a high-throughput, selective bioanalytical method based on LC/MS/MS that enables sample preparation to recover amyloids at pg/mL levels in the presence of high concentrations of interfering proteins and peptides. Peptide.
Although the time required to develop an immunoassay is acceptable during later drug development, it is almost impractical at an earlier stage; in this case, if there is a high throughput that can quantify multiple peptides and A reliable method is expected.
This work focused on the development of LC, MS and selective SPE sample preparation methods for 1-38, 1-40 and 1-42 fragments of amyloid precursor protein (APP) to support preclinical studies. The use of a single, high-throughput method for the analytical determination of various Aβ peptides without the need for time-consuming immunoprecipitation steps was successfully developed and validated. In particular, Aβ-like peptides have many unique analytical challenges, including non-specific binding, poor solubility, aggregation, and low sensitivity to mass spectrometry. The steps taken at each stage of method development minimize or eliminate the impact of these problems.
With the advent of AD disease remission strategies, quantitative analysis of multiple Aβs that may be associated with AD symptoms in addition to Aβ 38, 40, and 42 may help provide more insight into the disease and its development process. It is also possible to modify the methods described herein to make them suitable for quantitative analysis of those peptides.
淀粉 amyloid 1-38 peptide, molecular weight 4132 , pI 5.2
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGG
淀粉 amyloid 1-40 peptide, molecular weight 4330 , pI 5.2
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
β-amyloid 1-42 peptide, molecular weight 4516, pI 5.2
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA
Figure 1: Amino acid sequence and pI data for beta amyloid peptides 1-38, 1-40 and 1-42
experiment
Conditions of the UPLC ® method
Column: ACQUITY UPLC ® BEH C 18 , 300 Å, 2.1 x 150 nm, 1.7 μm
Mobile phase: A: 0.3% NH 4 OH (by volume) of aqueous solution
B: 90% acetonitrile, 10% mobile phase A
Gradient: 90% A for 1 minute, reduced to 55% A in 5.5 minutes and held for 0.2 minutes, then returned to the initial level
Flow rate: 0.2 mL/min
Injection volume: 10μL
Temperature: 50 ° C
Mass spectrometry condition
System: Waters Xevo TM TQ Triple Quadrupole Mass Spectrometer, operating in ESI+MRM mode
Desolvation gas flow rate: 800 L / hour
Source temperature: 120 ° C
Desolvation temperature: 450 ° C
Collision chamber pressure: 2.6 × 10 (-3) mbar
MRM transition states and conditions: see Table 1
Sample pretreatment
200 μL of cerebrospinal fluid (human cerebrospinal fluid, monkey cerebrospinal fluid or spiked artificial cerebrospinal fluid + 5% rat plasma) was diluted with 1:1 of 5 M guanidine hydrochloride and shaken at room temperature for 45 minutes. Then, the sample was further diluted with 200 μL of a 4% H 3 PO 4 aqueous solution.
Note: For spiked samples, the sample can be allowed to equilibrate for 30 minutes at room temperature after standard addition and before dilution with guanidine hydrochloride.
Solid phase extraction (SPE )
Oasis ® MCX based on μElution 96-well
Pretreatment: 200 μL methanol
Equilibrium: 200 μL 4% H 3 PO 4 in water
Loading: 600 μL of pretreated sample
Cleaning 1:200 μL 4% H 3 PO 4 aqueous solution
Cleaning 2:10% ACN aqueous solution
Elution: 2 × 25 μL 75:15:10 ACN: water: NH 4 OH concentrated solution
Dilution: 25 μL of water
Injection volume: 20μL
Peptide name | Precursor ion | Product ion | Product ion ID | Cone hole voltage (V) | Collision energy (eV) |
Beta amyloid 1-38 peptide | 1033.5 | 1000.3 | b 36 | 33 | twenty three |
N15 internal standard of β-amyloid 1-38 peptide | 1046 | 1012.5 | | 30 | twenty two |
Amyloid 1-40 peptide | 1083 | 1053.6 | b 39 | 33 | 25 |
N15 internal standard of β amyloid 1-40 peptide | 1096 | 1066.5 | | 35 | twenty two |
Amyloid 1-42 peptide | 1129 | 1078.5 | b 40 | 28 | 30 |
N15 internal standard of β-amyloid 1-42 peptide | 1142.5 | 1091.5 | | 35 | 28 |
Table 1 : MRM transition states and mass spectrometry conditions for amyloid beta peptide and its N15- labeled internal standard
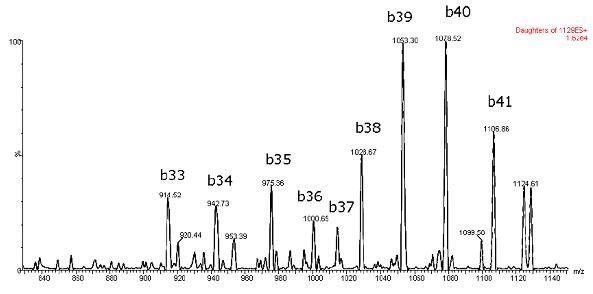
Figure 2: Typical ESI+MS/MS spectrum of beta amyloid 1-42 peptide
Results and discussion
The biggest challenge in developing these methods is to overcome the problems of solubility, adsorption and aggregation and to obtain sufficient selectivity and sensitivity to meet the requirements of this application. Proper mobile phase and injection solvent composition and the wise choice of SPE elution solvent are just a few of the key factors in dealing with these issues.
Mass Spectrometry
Mass spectrometry was performed in positive ion mode because the CID of the 4+ precursor produced several different product ions corresponding to the intrinsic specific b-sequence ions (typical spectra are shown in Figure 2). MS/MS in negative ion mode showed significant water loss. Figure 3 gives an example of the specific differences between the two methods. Although the overall sensitivity to the negative ion mode is higher for solvent standards, the sensitivity advantage of negative ion mode is diminished in the presence of matrix, while the specificity and signal-to-noise ratio improvement in positive ion mode is decisive for accurate quantification in cerebrospinal fluid samples. effect.
Ultra performance liquid chromatography
Figure 4 shows the separation of these three amyloid peptides. Although the exact percentage of NH 4 OH in the mobile phase is critical to negative ion sensitivity, the signal in the ESI+ mode has been shown to be more robust to subtle changes in the mobile phase, allowing the LC/Autosampler to be at least 24 hours. It remained stable during the above time period. In contrast, more than 50% or of 10-12 hours after ESI- signal due to natural variations in mobile phase NH 4 OH concentration (volatilization) loss. This further emphasizes the robustness of the ESI+MS method.
Solid phase extraction (SPE )
SPE is carried out using Oasis ® MCX (a mixed mode adsorbent) to enhance the selectivity of the extraction process. The adsorbent also relies on reverse phase and ion exchange retention mechanisms to selectively separate the beta amyloid peptide component from other high abundance polypeptides in complex cerebrospinal fluid samples. The use of a specific 96-well Oasis ® μElution provides significant concentration without the need for solvent evaporation and reconstitution, minimizing peptide loss. Furthermore, peptide binding by ion exchange provides orthogonality to the overall process.
During the initial method development process, a large amount of non-specific binding (NSB) was observed when extracting artificial cerebrospinal fluid. We added 5% rat plasma (with a different beta amyloid peptide sequence) to eliminate NSB.
SPE is one of the more important aspects of the whole process. The extremely high selectivity of the amyloid component plus the resolution of the UPLC at standard flow rates enables ultrafast analysis of preclinical samples.
Linearity, accuracy and precision
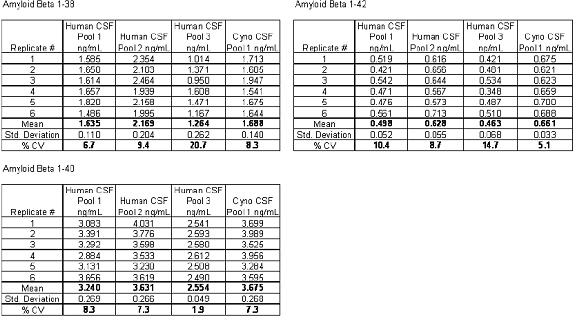
An N15-labeled internal standard was used for each peptide. For the aliquots of 0.1-10 ng/mL artificial cerebrospinal fluid + 5% rat plasma, the standard curves of the three beta amyloid peptides were linear. A typical standard curve for the beta amyloid 1-38 peptide is shown in Figure 5. Baseline levels of amyloid peptide were quantified based on two standard curves obtained using both spiked human cerebrospinal fluid and "artificial cerebrospinal fluid + 5% rat plasma", and there was no statistically significant difference in the calculated values ​​at baseline levels. Artificial cerebrospinal fluid is chosen because it is inexpensive and is a relatively easy substrate. The baseline levels of the beta amyloid 1-42 peptide extracted from three human cerebrospinal fluids and one monkey cerebrospinal fluid are shown in FIG. The statistical results of the baseline level measurements of all three amyloid beta peptides are shown in Table 2.
Excess spiked QC samples of 0.2, 0.8, 2 and 6 ng/mL were prepared using 3 human cerebrospinal fluid mixed samples and 1 monkey cerebrospinal fluid mixed sample. Accuracy and accuracy values ​​are in accordance with the control criteria for LC/MS/MS measurements. Typical results of quality control sample analysis are shown in Table 3.

Figure 3: Comparison of mass spectrometry specificity of electrospray mode using negative ions (dehydration product ions) or positive ions (product ions of b-sequence ions)
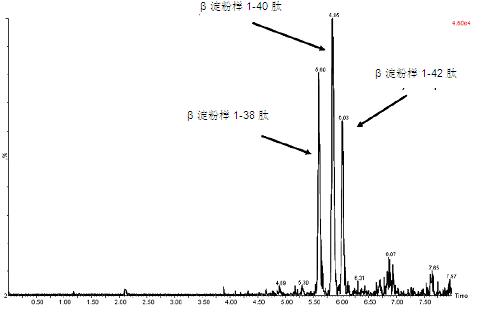
Figure 4: UPLC/MS/MS analysis of beta amyloid 1-38, 1-40 and 1-42 peptides extracted from artificial cerebrospinal fluid + 5% rat plasma
Compound Name: 1-38
Correlation coefficient r=0.996956, r 2 =0.993921
Calibration curve: 0.333755*X+-0.0119724
Response type: internal standard (reference item 2) area* (internal standard concentration / internal standard area)
Curve type: linear; source: single; weight: 1/x; horizontal axis: none
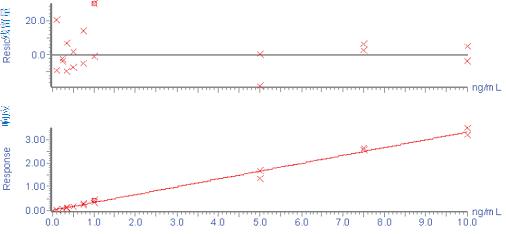
Figure 5: Typical standard curve of β-amyloid 1-38 peptide extracted from artificial cerebrospinal fluid + 5% rat plasma
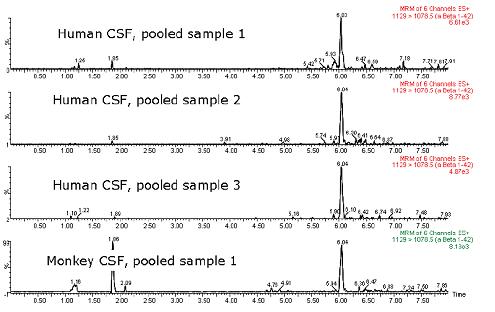
Figure 6: Typical chromatogram showing baseline levels of beta amyloid 1-42 peptide in three human cerebrospinal fluids and one monkey cerebrospinal fluid
Table 2: Baseline levels of amyloid beta peptide in a mixture of three human cerebrospinal fluid samples and one monkey cerebrospinal fluid sample
Beta amyloid 1-38 peptide
Excess spike concentration Ng/mL | Control concentration | Average calculated concentration | standard deviation | % CV | Number of passes for repeated tests | Average accuracy |
| | | | | | |
0.2 | 1.49 | 1.355 | 0.071 | 5 | 3/3 | 90.9 |
0.8 | 2.09 | 1.843 | 0.118 | 6 | 3/3 | 88.2 |
2 | 3.29 | 3.287 | 0.319 | 10 | 3/3 | 99.9 |
6 | 7.29 | 7.701 | 0.478 | 6 | 3/3 | 105.6 |
Amyloid 1-40 peptide
Excess spike concentration Ng/mL | Control concentration | Average calculated concentration | standard deviation | % CV | Number of passes for repeated tests | Average accuracy |
| | | | | | |
0.2 | 2.75 | 2.359 | 0.015 | 1 | 2/3 | 85.8 |
0.8 | 3.35 | 3.054 | 0.016 | 1 | 2/3 | 91.2 |
2 | 4.55 | 3.929 | 0.011 | 0 | 3/3 | 86.4 |
6 | 8.55 | 8.209 | 0.5 | 6 | 3/3 | 96 |
Amyloid 1-42 peptide
Excess spike concentration Ng/mL | Control concentration | Average calculated concentration | Standard deviation | % CV | Number of passes for repeated tests | Average accuracy |
| | | | | | |
0.2 | 0.663 | 0.655 | 0.07 | 11 | 3/3 | 98.8 |
0.8 | 1.263 | 1.145 | 0.058 | 5 | 3/3 | 90.7 |
2 | 2.463 | 2.403 | 0.121 | 5 | 3/3 | 97.5 |
6 | 6.463 | 5.986 | 0.701 | 12 | 3/3 | 92.6 |
Table 3: Typical results of analysis of quality control samples prepared from three human mixed cerebrospinal fluids
in conclusion
1. We developed and validated SPE-LC/MS/MS bioanalytical methods for simultaneous quantitative analysis of various beta amyloid peptides in human and monkey cerebrospinal fluid.
2. Combining the high-selective extraction method based on μElution mixed mode SPE with the resolution of UPLC chromatographic analysis is the key to accurate, accurate and reliable quantitative analysis of three major β amyloid peptides in human and monkey cerebrospinal fluid. .
3. The use of positive ion MS/MS and b ion sequence fragments provides the mass spectrometry specificity required for this application.
4. The extraction of 96 samples and preparation of the injection can be completed in less than 30 minutes, thus meeting the sample preparation flux requirements required for preclinical studies.
5. The methods described herein avoid time-consuming immunoassays or immunoprecipitation steps in preclinical research work.
6. The mass range and sensitivity of the Xevo TQ mass spectrometer allows for the selection of high m/z precursors for fragmentation and the selection of highly specific b-ion fragments, which increases the signal-to-noise ratio of this assay and overall increases its specificity.
7. Such methods may also allow for the simultaneous, specific, and high-throughput method of simultaneous determination of several different amyloid beta peptides in a sample while still achieving low concentrations of endogenous beta amyloid High sensitivity required for peptide analysis. This is a significant advantage because ELISA assays require multiple assays using multiple antibodies.
Selected references
1. TA Lanz, JB Schachter. Journal of Neuroscience Methods, 169 (2008) 16-22.
2. T. Oe et al. Fast Communications in Mass Spectrometry, 20 (2006) 3723-3735.
3. JR Slemmon et al. Journal of Chromatography: Bioanalysis, 846 (2007) 24-31.
4. NT Ditto et al. Journal of Neuroscience Methods, 182 (2009) 260-265.
5. TA Lanz, JB Schachter. Journal of Neuroscience Methods, 157 (2006) 71-81.
6. MJ Ford et al. Journal of Neuroscience Methods, 168 (2008) 465-474.
7. E. Portelius et al. Journal of Proteomics Research, 6 (2007) 4433-4439.
Acknowledgement
The authors wish to express their gratitude to Wenlin Li (Pfizer PDM) for her preliminary work on the use of immunoaffinity LC/MS/MS for the analysis of beta amyloid peptides.
Waters Corporation
34 Maple Street, Milford, MA, 01757
Tel: (508) 478-2000; Fax: (508) 478-1990
Http://
As the leading textile enzyme manufacturer and supplier, we are committed to
bringing innovative textile solutions to the market with good product stability
and performance by combining world-class production and application
technologies to best satisfy our customer needs. The desizing products for the
applications under different condition are available to fit various
processing requirement.
De-sizing
Textile auxilliary agent,Desizing Enzymes,amylase
Sunson Industry Group Co., Ltd , https://www.sunsonchinaenzymes.com