Anticoagulant Rivaroxaban Market Analysis
On June 22, 2012, the FDA refused to approve the application of Johnson & Johnson and Bayer's rivaroxaban (trade name: Xarelto, English name: Xarelto) for patients with acute coronary syndrome to prevent heart attack and stroke . Johnson & Johnson has the right to sell rivaroxaban in the United States while Bayer has the right to sell rivaroxaban in Europe.
Rivaroxaban was developed by Bayer Pharmaceuticals of Germany and was listed on the European Union on September 30, 2008. The first approval of its use in patients undergoing selective hip or knee replacement surgery to prevent venous thromboembolism (VTE). The formation of thrombus is an important pathogenic factor for cardiovascular diseases such as myocardial infarction, stroke, deep vein thrombosis, pulmonary embolism, etc. Antithrombotic therapy has always been the core of the rescue measures and prevention strategies for such diseases, among which anticoagulation is an antithrombotic treatment. One of the important methods. Since the discovery of heparin in 1914, anticoagulants have played an important role in the prevention and treatment of life-threatening thromboembolic events.
Rivaroxaban is a highly selective, oral drug that directly inhibits factor Xa. By inhibiting factor Xa, the endogenous and exogenous pathways of coagulation cascades can be interrupted and thrombin generation and thrombosis can be inhibited.
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is one of the leading causes of death in humans. Deep venous thrombosis is a common complication after major orthopaedic surgery. Its main hazards can make the affected limb swell, affect activities, and can cause disability in severe cases. More importantly, 90% of pulmonary embolisms are caused by DVT emboli fall off. fatal. For total hip replacement, DVT is prone to occur after replacement. With the popularity of total hip arthroplasty in China, the problem of DVT after replacement has become increasingly prominent.
At present, Rivaroxaban has gradually replaced the old anticoagulant warfarin in the European market. According to the competition pattern of the intranet-clinical antithrombotic drugs, Rivaroxaban entered the domestic market by Bayer in 2009 and was ranked 19th in the market of antithrombotic drugs in that year, which proved the market of Rivaroxaban. The potential is amazing.
The rankings of rivaroxaban and warfarin in the antithrombotic drug market in the past three years. Name of the product in 2009. Ranking in 2010. Ranking in 2011. Rank Rivasaban 19th, 14th, and 12th
Warfarin 14th 16th 18th
(Data source: Competition network for clinical drug use at Ricenet)
Due to the late listing of rivaroxaban, there are no companies in the country currently reporting on new drugs. Whether it will become a heavy drug like Polivi will still need to be tested in the market.
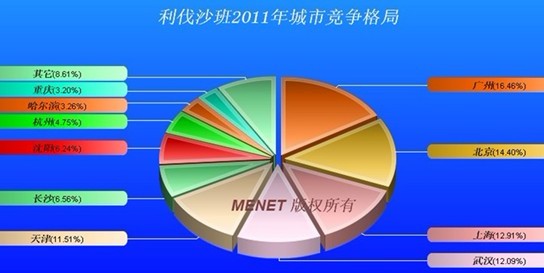
According to the data of the city's competitive landscape in 2011, Rivaroxaban has average sales across the country, with Guangzhou, Beijing, Shanghai, Wuhan, and Tianjin all exceeding a market share of 10% and Guangzhou with a maximum of 16.46%. (Researcher lily-cha)
Rivaroxaban was developed by Bayer Pharmaceuticals of Germany and was listed on the European Union on September 30, 2008. The first approval of its use in patients undergoing selective hip or knee replacement surgery to prevent venous thromboembolism (VTE). The formation of thrombus is an important pathogenic factor for cardiovascular diseases such as myocardial infarction, stroke, deep vein thrombosis, pulmonary embolism, etc. Antithrombotic therapy has always been the core of the rescue measures and prevention strategies for such diseases, among which anticoagulation is an antithrombotic treatment. One of the important methods. Since the discovery of heparin in 1914, anticoagulants have played an important role in the prevention and treatment of life-threatening thromboembolic events.
Rivaroxaban is a highly selective, oral drug that directly inhibits factor Xa. By inhibiting factor Xa, the endogenous and exogenous pathways of coagulation cascades can be interrupted and thrombin generation and thrombosis can be inhibited.
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is one of the leading causes of death in humans. Deep venous thrombosis is a common complication after major orthopaedic surgery. Its main hazards can make the affected limb swell, affect activities, and can cause disability in severe cases. More importantly, 90% of pulmonary embolisms are caused by DVT emboli fall off. fatal. For total hip replacement, DVT is prone to occur after replacement. With the popularity of total hip arthroplasty in China, the problem of DVT after replacement has become increasingly prominent.
At present, Rivaroxaban has gradually replaced the old anticoagulant warfarin in the European market. According to the competition pattern of the intranet-clinical antithrombotic drugs, Rivaroxaban entered the domestic market by Bayer in 2009 and was ranked 19th in the market of antithrombotic drugs in that year, which proved the market of Rivaroxaban. The potential is amazing.
The rankings of rivaroxaban and warfarin in the antithrombotic drug market in the past three years. Name of the product in 2009. Ranking in 2010. Ranking in 2011. Rank Rivasaban 19th, 14th, and 12th
Warfarin 14th 16th 18th
(Data source: Competition network for clinical drug use at Ricenet)
Due to the late listing of rivaroxaban, there are no companies in the country currently reporting on new drugs. Whether it will become a heavy drug like Polivi will still need to be tested in the market.
According to the data of the city's competitive landscape in 2011, Rivaroxaban has average sales across the country, with Guangzhou, Beijing, Shanghai, Wuhan, and Tianjin all exceeding a market share of 10% and Guangzhou with a maximum of 16.46%. (Researcher lily-cha)

Window-Frame Transparent Dressing
Window-Frame Transparent Dressing,Oem Shear Transparent Dressing,Custom Transparent Dressing,Waterproof Transparent Dressing
Zhende Medical Co.,Ltd , https://www.zdmedicalproduct.com